Bloodborne Pathogens And Needlestick Prevention
Overview
What are bloodborne pathogens?
Bloodborne pathogens are infectious microorganisms in human blood that can cause disease in humans. These pathogens include, but are not limited to, hepatitis B , hepatitis C and human immunodeficiency virus . Needlesticks and other sharps-related injuries may expose workers to bloodborne pathogens. Workers in many occupations, including first responders, housekeeping personnel in some industries, nurses and other healthcare personnel, all may be at risk for exposure to bloodborne pathogens.
What can be done to control exposure to bloodborne pathogens?
In order to reduce or eliminate the hazards of occupational exposure to bloodborne pathogens, an employer must implement an exposure control plan for the worksite with details on employee protection measures. The plan must also describe how an employer will use engineering and work practice controls, personal protective clothing and equipment, employee training, medical surveillance, hepatitis B vaccinations, and other provisions as required by OSHA’s Bloodborne Pathogens Standard . Engineering controls are the primary means of eliminating or minimizing employee exposure and include the use of safer medical devices, such as needleless devices, shielded needle devices, and plastic capillary tubes.
General Guidance
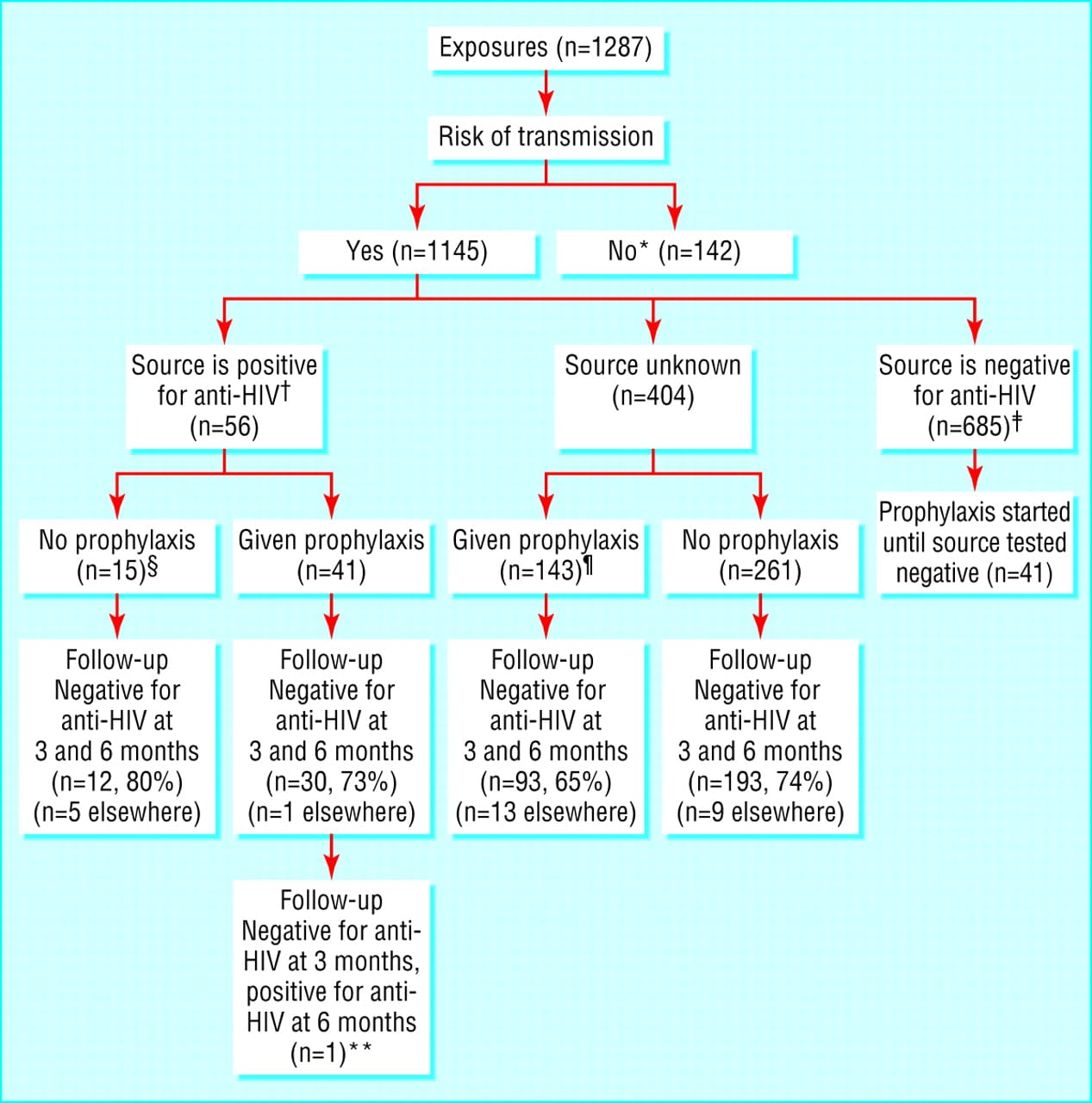
Sample Collection And Laboratory Analysis
From each participant, 5 ml of venous blood was drawn aseptically. A rapid screening was done for HBV surface antigen and anti-HCV antibodies using immunochromatographic assay on the plasma sample as per the manufacturer’s instruction. The remaining samples were packed in a leak-proof triple container and transported to THRI at room temperature on the same day of collection.
Samples, which were positive for HBsAg and anti-HCV on the immunochromatographic assay, were confirmed with HBsAg and anti-HCV Enzyme-Linked Immuno-sorbent assay at THRI, respectively. The HBsAg/anti-HCV ELISA is a highly specific and sensitive fourth-generation qualitative serological test that detects both antigens and antibodies. ELISA tests were performed under sterile conditions. Positive and negative controls were used and the cut-off values for the respective tests were defined according to the manufacturers instructions.
HIV-1 RNA viral load was quantified using reverse transcription PCR. Briefly, HIV-1 RNA was extracted from 0.2 ml of plasma sample on the Abbott m2000sp automated sample preparation system . Extracted RNA was then measured using Abbott m2000rt quantitative Real-Time HIV-1 assay with HIV-1 RNA detection level of 40 to 10 million copies/ml based on the manufacturer’s procedures.
Infection Control Principles And Practices For Local Health Agencies
Bloodborne pathogens are microorganisms that cause disease and are present in human blood. They include but are not limited to human immunodeficiency virus , hepatitis B virus , and hepatitis C virus .
OSHA issued the Bloodborne Pathogens Standard 29 CFR Part 1910.1030to protect health care workers and others who come in contact with blood and other potentially infectious material during their occupational duties. The purpose of the standard is to prevent occupational exposure to bloodborne pathogens and to reduce the chances of infection when exposure does occur.
This standard requires employers to write and implement an exposure control plan for employees with occupational exposure to blood and OPIM, using administrative, engineering, and work practice controls to prevent or minimize employee exposure.
The exposure control plan must contain at least the following elements:
Frequently Asked Questions
What staff members are required to be in a bloodborne pathogens exposure control plan?
How often do we have to train staff?
All staff need to have training at the time they are initially assigned duties with occupational exposure, and annually thereafter. Training must be at the educational level and in the language of the employee.
What records do we need to keep?
Training records, which are kept for three years from the date on which the training occurred, and medical records which are kept for the duration of employment plus 30 years .
Who can perform the training?
You May Like: Yeast Infection Treatment While Breastfeeding
Hiv/hbv And/or Hcv Infections And Hepatotoxicity In Pregnant And Non
Keywords:Hepatitis B virus, HBV, Hepatitis C virus, HCV, hepatotoxicity, human immune deficiency virus, infection
Important Note: All contributions to this Research Topic must be within the scope of the section and journal to which they are submitted, as defined in their mission statements. Frontiers reserves the right to guide an out-of-scope manuscript to a more suitable section or journal at any stage of peer review.
Keywords:Hepatitis B virus, HBV, Hepatitis C virus, HCV, hepatotoxicity, human immune deficiency virus, infection
Important Note: All contributions to this Research Topic must be within the scope of the section and journal to which they are submitted, as defined in their mission statements. Frontiers reserves the right to guide an out-of-scope manuscript to a more suitable section or journal at any stage of peer review.
How Are Hepatitis B And Hepatitis C Spread From Person To Person
Like HIV, the hepatitis B and hepatitis C viruses spread:
- Perinatally: Pregnant people can pass these infections to their infants. HIV-HCV coinfection increases the risk of passing on hepatitis C to the baby.
- Sexually: Both viruses can also be transmitted sexually, but HBV is much more likely than HCV to be transmitted sexually. Sexual transmission of HCV is most likely to happen among gay and bisexual men who have HIV.
Also Check: Signs Of Hernia Mesh Infection
Expression Pattern Of Mirrnas In Hcv Cases And Controls
The current study reported a significant difference between patients and controls regarding miRNAs level where the levels of serum miR-122 and miR-155 were significantly higher in the chronic hepatitis C patients than in healthy controls, On the other hand, the levels of serum miR-196 and miR-29 were significantly lower in the chronic hepatitis C patients than in healthy controls .
Fig. 1
Expression pattern of mirRNAs in HCV cases and controls: Data are expressed as mean, SD, median and were analyzed by MannWhitney U test. The data was statistically significant
Hiv And Hepatitis B And Hepatitis C Coinfection
Hepatitis B and hepatitis C are liver infections caused by a virus. Because these infections can be spread in the same ways as HIV, people with HIV in the United States are often also affected by chronic viral hepatitis.
Viral hepatitis progresses faster and causes more liver-related health problems among people with HIV than among those who do not have HIV. Liver disease, much of which is related to HBV or HCV, is a major cause of non-AIDS-related deaths among people with HIV.
Given the risks of hepatitis B or hepatitis C coinfection to the health of people with HIV, it is important to understand these risks, take steps to prevent infection, know your status, and, if necessary, get medical care from a health care provider who is experienced in treating people who are coinfected with HIV and HBV, or HIV and HCV.
Also Check: Can A Yeast Infection Cause Chlamydia
Study Selection And Data Extraction
Two authors independently assessed titles and abstracts for eligibility, and any disagreement was resolved through discussion. A copy of the full text was obtained for all research articles that were available and approved in principle to be included. Abstraction of data was in accordance of the task separation method method and result sections in each study were separately abstracted in different occasions to reduce bias . Moreover, data abstraction was conducted with no consideration of authors qualifications or expertise. Each research article was screened for all relevant information and recorded in the data extraction file , as one article may report outcome of several prevalence studies prevalence data of HIV, HBV or HCV for a defined population group, in a defined region, or combine two or more of them or provide specific prevalence in one infection under study as Occult Hepatitis B . Moreover, data from each method section was extracted using a predefined set of variables study characteristics, type of participants, study population size, geographical region and the screening protocol used.
Resources For Drug Interaction
Most persons with HIV infection are taking multidrug antiretroviral therapy, which may pose a problem with drug interactions when initiating HCV treatment. For resources on drug interactions that may occur with HIV antiretroviral medications and HCV treatment medications, access the following sites:
- AASLD-IDSA HCV Guidance: Patients with HIV/HCV Coinfection
Read Also: Can You Get Urinary Tract Infection Medication Over The Counter
Expression Levels Of Mirna In Svr And Nr
The expression levels of miR-122 and miR-155 were significantly higher in non-responders to DAAs than in responders. In contrast, the expression levels of miR-29 and miR-196 were significantly higher in responders to DAAs than in non-responders .
Fig. 3
Serum miRNA expression levels in SVR and NR: a miRNA 155, b miRNA 122, c miRNA 29, d miRNA 196. The box represents the 2575% percentiles the line inside the box represents the median and the upper and lower lines representing the 1090% percentiles of pretreatment expression levels of a miR-122, b miR-155, c miR-29, d miR-29 in SVR and NR . Data were analyzed by MannWhitney U test. The data was statistically significant
Detection And Diagnosis Of Hiv Infection
After initial primary infection with HIV, there is a window period prior to the development of detectable antibody. In persons with known exposure dates, the estimated median time from initial infection to the development of detectable antibody is 2.4 months 95% of individuals develop antibodies within 6 months of infection . Among HCWs with a documented seroconversion to HIV, 5% tested negative for HIV antibodies at > 6 months after their occupational exposure but were seropositive within 12 months . The two antibody tests commonly used to detect HIV are the enzyme immunoassay and the Western blot. An HIV test result is reported as negative when the EIA result is negative. The result is reported as positive when the EIA result is repeatedly reactive and when the result of a more specific, supplemental confirmatory test, such as the Western blot, is also positive. Once an individual develops an antibody response, it usually remains detectable for life. HIV infection for longer than 6 months without detectable antibody is uncommon .
Read Also: You Realize Your Computer Has Been Infected With Malware
Prevalence Among Pregnant Women
Five studies reported HIV prevalence among pregnant women in Sudan, one study was conducted in Kassala, Eastern Sudan , three in Khartoum State , and one in Gadarif State . The prevalence rates ranged from 0 to 1.4%. The result of meta-analysis showed overall pooled prevalence of 0.4% , the heterogeneity was I2=56%.
Two studies have been found concerned of the prevalence of HBsAg among pregnant women in Sudan Elsheikh and colleagues study was a cross sectional conducted at Omdurman maternity hospital in Khartoum State in 2007 . HBsAg was detected in 41 out of 728 tested women. Later on, in 2014, 20 out of 396 tested pregnant women were concluded to be positive for HBsAg in the study of Osman and colleagues . The pooled prevalence was 5.5% , the heterogeneity was I2=0%.
The same two studies have been identified establishing the prevalence of HCV antibodies among pregnant women. Elsheikh and colleagues reported low HCV prevalence of among pregnant women in Khartoum .While a higher prevalence of has been estimated in Wad Madani city among the same population . The pooled prevalence was 0.6% , with heterogeneity value of 0%.
Preventing Hepatitis And Hiv Infections In The Hospital

Isolation precautions create barriers between people and germs. They help prevent the spread of germs in the hospital.
Follow standard precautions with all people.
When you are near or are handling blood, bodily fluids, body tissues, mucous membranes, or areas of open skin, you must use personal protective equipment . Depending on the exposure, you may need:
Read Also: Can I Get A Yeast Infection From Sex
General Population And Populations At High Risk
All authors – after comprehensive discussion – approved the following four domains to be considered as possible sources of selection bias in general population studies and studies toward specific populations at risk: age, gender, population coverage and sample size. Studies conducted in Female Sex Workers were not assessed for gender bias. Points were given for representativeness or a lower risk of bias in each domain . A total score for risk of bias was calculated by adding up the scores in all four domains, resulting in a score of between 0 and 7. The highest score indicates the lowest risk of bias, studies in the general population or those toward specific populations at risk with a score for risk of bias greater or equal to 3 were included in the review. All authors agreed that the four domains are the only sources of bias a reader can determine inclusion of different age groups, inclusion of both sexes, conduction of the study in different regions/locations and more study participants were considered vital to reduce the selection bias to the minimum.
Table 1 Assessment of quality of general population studies and studies toward specific Populations at risk
Study Populations And Recruitment
All HIV patients on ART were our study populations. Providing written informed consent or assent, being an age of3 years, and being on ART for at least 6 months at the start of the study were the inclusion criteria. Patients who were critically ill, those vaccinated for HBV, and those treated for HBV and/ or HCV were excluded. Finally, 439 patients were enrolled in the study conveniently. Subsequently, data on socio-demographic and clinical characteristics of the study participants were collected using a structured questionnaire and a standardized recording format, respectively.
Also Check: Why Do I Get Sinus Infections So Much
Prevalence By Studies Publication Time
Regarding HIV the earliest study among studies included in the review has been found to be published in 1997 with prevalence rate of 1.2% , followed by one study in 2006 with prevalence rate of 1% , In 2010, two studies were published with prevalence rates of 5.7% and 0.9% . The pooled prevalence was 2.8% , the heterogeneity was I2=78%. In 2011 one study as well reported prevalence rate of 0.2% . Followed by another two studies in 2012 with prevalence data of 18.3% and 3% . The pooled prevalence was 10.2% , the heterogeneity was high . In 2013, the multi-centre national study of Elhadi and colleagues was conducted with prevalence rate of 0.8%. Furthermore, one study was published in 2014 with prevalence rate of 1.5% . The next two years witnessed publication of two studies one in 2015 with prevalence rate 0.7% , and the other one in 2016 with prevalence rate of 0.4% .
Fig. 8
Prevalence rates of the three infections according to publication period of all studies included in the review. Data was plotted as line graphs, when there is no published study in a given year the line predicts the direction to be in accordance to the next year with published study/ies and get disrupted to indicate a missing data
Laboratory Investigations And Clinical Assays
Each individual had a sample of 10 ml of venous blood drawn that was taken under strict aseptic conditions. The supernatant serum from one portionwhich had been allowed to clotwas centrifuged and utilised for laboratory tests on the complete blood count , aspartate transferase , and albumin levels. The enzyme-linked immunosorbent test was used to measure antibodies for HCV, HBV, and HIV . Using quantitative real-time PCR , serum HCV RNA levels were assessed before to and six months after the conclusion of therapy. The tests were all conducted in accordance with the manufacturer’s instructions. Before starting treatment, abdomen ultrasonography was performed on all HCV patients to determine the degree of fibrosis.
Read Also: Is A Uti And Yeast Infection The Same Thing
How Does Drug Use Affect Symptoms And Outcomes Of A Viral Infection
Drug use can worsen the progression of HIV and its symptoms, especially in the brain. Studies show that drugs can make it easier for HIV to enter the brain and cause greater nerve cell injury and problems with thinking, learning, and memory. Drug and alcohol use can also directly damage the liver, increasing risk for chronic liver disease and cancer among those infected with HBV or HCV.
Roc Curve Analysis Of Serum Mirnas As Predictive Biomarker Differentiating Svr And Nr
All studied miRNAs could differentiate SVR from NR with an AUC of 0.973 for miR-122 , 0.878 for miR-155 , 0.808 for miR-29 and 0.874 for miR-196 , respectively. The optimal sensitivity and specificity to differentiate SVR from NR were for miR-122, for miR-155, for miR-29, for miR-196, respectively. Comparison of the ROC curve results suggested that the predictive accuracy of serum miR-122 was the most superior in the order of miR-122> miR-155> miR-196> miR 29 .
Fig. 5
ROC curve analysis of serum miRNAs as predictive biomarker differentiating SVR and NR: a miRNA 155, b miRNA 122, c miRNA 29, d miRNA 196
Read Also: Difference Uti And Yeast Infection
How Can You Prevent Hepatitis B And Hepatitis C
Hepatitis B: Vaccination is the best way to prevent all the ways that hepatitis B is transmitted. People with HIV who do not have active HBV infection should be vaccinated against it. The hepatitis B vaccine is now recommended for all infants, children and adults ages 19-59, as well as adults ages 60+ at high risk for infection. There is a 3-dose series of hepatitis B vaccine given over 6 months, and a 2-dose series given over 1 month. Additionally, there is a 2-dose combination vaccine that protects against both hepatitis A and hepatitis B.
Hepatitis C: No vaccine exists for HCV and no effective pre- or post-exposure prophylaxis is available. Injection drug use is one of the risk factors for hepatitis C. For people who inject drugs, the best way to prevent hepatitis C infection is to always use new, sterile needles or syringes, and never reuse or share needles or syringes, water, or other drug preparation equipment. Community-based prevention programs, such as medication-assisted treatment and syringe services programs provide support and services aimed at preventing and reducing the transmission of HCV. Although the risk of sexual transmission of HCV is considered to be low, avoiding unprotected sexual exposure by using condoms has been shown to reduce the chance of sexually transmitted infections.